Space research helps find targets for early Alzheimer’s test
Methods used to study asteroids and planets could be used in a new blood test for Alzheimer’s disease – potentially before clinical symptoms develop
First published by the University of Melbourne
By Dr Brandon Mahan, University of Melbourne and Professor Ashley Bush, The Florey and University of Melbourne
One of the hardest things about a diagnosis of Alzheimer’s disease (AD) is that by the time conventional testing is useful, the disease has already progressed.
By this time, a person is often already having difficulties with memory and thought processes that impact their daily lives and those of their loved ones.

Existing non-invasive AD tests rely on brain scans and protein-based blood tests to detect biomarkers and bodily changes indicative of the disease.
For example, Positron Emission Tomography (PET) images the build-up of insoluble protein clumps in the brain (amyloid beta), and protein-based blood tests detect abnormal concentrations of AD-related proteins (amyloid beta and tau) in the bloodstream.
While some protein-based blood biomarkers may offer early detection and higher test accessibility, they still rely on significant protein anomalies that may be more of a consequence than a cause of AD.
As the world’s population ages, the incidence of AD and other forms of dementia is rising. The number of dementia sufferers is predicted to double every 20 years, with the global cost of dementia forecast to cost US$2.8 trillion by 2030.
Dementia is the second leading cause of death in Australia and the leading cause for Australian women.
As there is currently no cure for AD and quality of life is based on early detection and treatments, detecting the disease as early as possible holds the key to alleviating its social and global burden.
A new approach to testing
Our latest study brought together researchers from Melbourne Analytical Geochemistry (MAG), working closely with neuroscientists at The Florey and geochemists at l’Institut de Physique du Globe de Paris.
We adapted highly sensitive techniques originally developed for cosmochemistry – to study the formation and evolution of the Earth, the Moon, other planets and asteroid samples – to instead search for early biomarkers of AD.

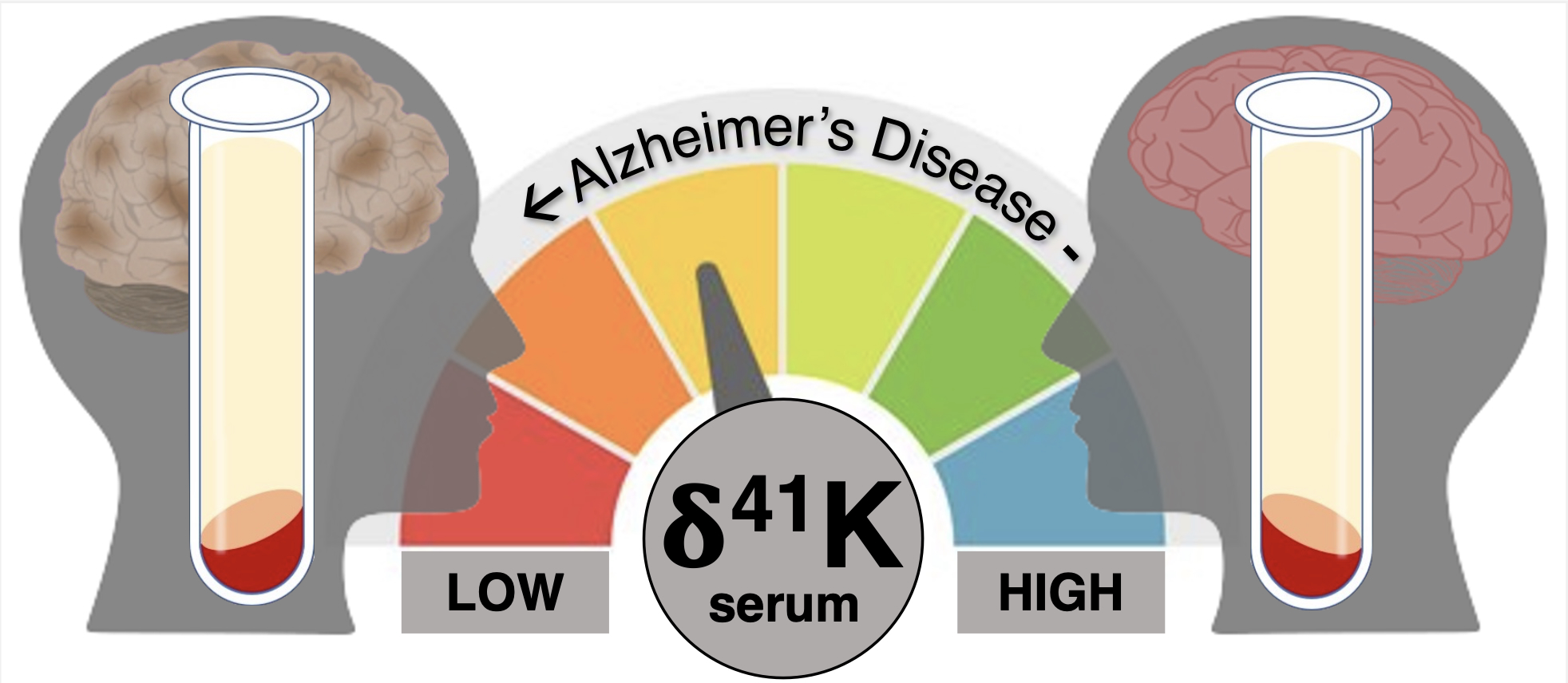
Our study found a difference we can reliably differentiate between healthy and AD patients using the difference in the relative levels of potassium isotopes (atoms of the same element with slightly different mass) in blood serum samples.
The new test is scalable and unlike protein-based diagnostics that can break down during storage, it avoids sample stability issues because the inorganic biomarker (potassium) is stable and does not need special storage or refrigeration.
Due to its ability to detect essentially instantaneous changes in body chemistry, we expect that the test could detect early stages of the disease before clinical symptoms appear.
Cosmochemistry and Alzheimer’s disease
In the last few decades, the fields of analytical geochemistry and cosmochemistry have seen technological advances that now allow for the analysis of extremely small changes in the relative proportions of isotopes for many elements on the periodic table.
You may recall learning about elements like potassium (K), Sodium (Na) and Calcium (Ca) at school. Isotopes are atoms with the same number of protons but different numbers of neutrons. They share the same chemical properties but differ slightly in mass.
Early phases of AD are known to change bodily function with respect to several elements, including potassium.
By using a type of analysis known as inorganic mass spectrometry, we can tell different isotopes of the same element apart by their mass.
In cosmochemistry, high resolution analyses of precious extraterrestrial samples – including asteroids and meteorites, as well as those from moons and other planets – require the absolute highest level of care and accuracy, where analyses must be done on the very smallest amounts of sample possible.
So, after recognising the many parallels with the preciousness of samples and the need for definitive accuracy, we realised we could apply this technique to biomarkers in Alzheimer's diesease development.
This small but powerful field of research, that applies analytical geochemistry to medical research, is called ‘isotope metallomics’.
While our team is currently focussing on Alzheimer’s disease, this approach has shown promise in other neurodegenerative diseases (eg Parkinson’s, ALS) as well as in cancer and osteoporosis detection.

AD changes in brain chemistry are echoed in the blood
The work on potassium isotopes had its genesis in research conducted through The Florey nearly a decade ago, which reported a drop in brain potassium levels at the same time as a rise in blood serum potassium levels.
Our hypothesis first put forward in 2022, was that if potassium concentrations are dropping in the brain while rising in blood serum, this could mean brain potassium is transferred into the blood, and a linked change in potassium isotope compositions could be accessible via a blood test.
The AD brain builds up certain metals (eg copper, zinc, iron), while purging others like potassium.
In earlier work, our team observed that although the level of potassium was reduced overall in the brain, the potassium that was detected had a higher proportion of the heavier K isotope (41K, atomic mass = 41) relative to the lighter K isotope (39K, atomic mass = 39) in comparison to healthy brain tissue.
This indicated that light K isotopes (39K) may be flushing out of the AD brain into the bloodstream, and if so, perhaps higher relative levels of the lighter K isotope in the blood serum of AD patients could be detected.
We tested this hypothesis using human blood samples from the Australian Imaging, Biomarker and Lifestyle (AIBL) Flagship Study of Ageing, one of the world’s largest and most comprehensive longitudinal AD research programs.
Through AIBL we explored potassium isotope compositions in a pilot cohort of healthy and AD-affected individuals. We found potassium isotopes readily distinguished healthy individuals from those affected by AD.
Despite the preliminary nature of this pilot study, this potential biomarker already performs at a level comparable to protein-based blood diagnostics. The approach is robust in terms of laboratory and analytical workflows and has accessibility and scalability potential.

Expanding the study
A top priority is to further explore this potential biomarker in larger studies, and different populations.
We are currently optimising instrumentation within Melbourne Analytical Geochemistry to simplify logistics for in-house analyses, which will essentially double the collective throughput across the partner facilities (University of Melbourne, and l’Institut de Physique du Globe de Paris where pilot analyses were conducted).
In addition to this expanded potassium isotope work, we have plans to extend the approach and explore isotope signatures for other metals in blood implicated in early AD, like copper, iron and zinc.
We are currently seeking collaborative partners and support to expand this project and further develop these inorganic, isotope-based biomarkers for Alzheimer’s and other diseases.




