Enlisting nanoparticles in the fight against superbugs
As antibiotics become less and less effective, researchers are turning to new ways to defeat drug-resistant bacteria like Golden Staph.
First published by the University of Melbourne
By Erin Munro
Andrea O’Connor and her University of Melbourne research team are at the forefront of one of the biggest battles in science today.
Their development of nanoparticles that can fight some of the most dangerous antibiotic-resistant bacteria is groundbreaking work, offering a way to fight infections at their source. It’s an important step towards managing the threat of antimicrobial resistance.

Scottish scientist Alexander Fleming famously discovered penicillin, the world’s first antibiotic, nearly a century ago in 1928. In doing so, he saved innumerable lives, rendering diseases that were previously deadly treatable with a course of medication.
But today the World Health Organization says antibiotic-resistant bacteria – bacteria that have developed a resistance to drugs, commonly referred to as ‘superbugs’ – are increasingly one of the biggest threats to global health, food and development.
And while bacteria have acquired this resistance on their own, it’s being fast-tracked through the over-prescription and misuse of antibiotics, as well as by poor hygiene practices.
Reports suggest there are approximately 700,000 deaths around the world each year resulting from antibiotic-resistant infections. It’s been predicted that figure will rise to 10 million per year by 2050.
Enter Associate Professor O’Connor, a chemical engineer by background and Deputy Head of the University’s School of Chemical and Biomedical Engineering. She works in the field of biomaterials, implants and tissue engineering (the practice of merging scaffolds – tiny, porous devices that act as a template to regenerate tissue and organs – with cells to repair wounds and damaged tissues).
It’s an area she entered via a twist of serendipity almost two decades ago, when a surgeon from St Vincent’s Hospital knocked on her office door, looking for someone who knew how to make polymer scaffolds to enhance cell growth in tissue engineering.
“He had read in a recent publication that they were useful,” Associate Professor O’Connor says.
“We’d never done it before but we thought it sounded very exciting. So, from that, we started a collaboration with researchers at the O’Brien Institute, which is part of St Vincent’s Hospital, and we’ve been working with them for almost 20 years.”
Since that fateful meeting, much of Associate Professor O’Connor’s research has focused on tissue engineering, and the scaffolds or sponges that are implanted to encourage new tissue growth.
“Eventually, that material that we implanted would degrade away,” she says. “But if that takes months or even up to a year, there’s a risk of infection forming on that device.”
These infections can pose significant health problems, and can also occur when medical implants such as heart valves and hip implants are inserted.
“Clinicians tell us infection leads to significant numbers of failures of medical devices and implants,” Associate Professor O’Connor adds.
“Increasingly, through our involvement in the field, we’ve realised it’s a major challenge.”
And so Associate Professor O’Connor and her team began researching antimicrobial materials – materials that impede the ability of microorganisms, such as bacteria, to grow. Having backgrounds in nanotechnology, the researchers worked on developing nanoparticles that would be effective against a wide range of infections.
“It was partly triggered by a collaborator who came to my lab from the US, Dr Phong Tran, who’s now at Queensland University of Technology,” says Associate Professor O’Connor.
“He has a background working with nanoparticles, and so that expertise boosted our interest and activity in that area.”
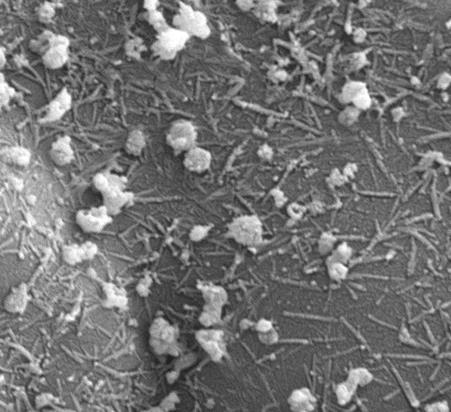
Together, the team found that selenium (a mineral that humans require in their diets to boost immunity and aid metabolism) in the form of nanoparticles can stop the growth of bacteria such as ‘Golden Staph’ (Staphylococcus aureus). Their findings are published in the Journal of Colloid and Interface Science.
They think the nanoparticles do this by disrupting the membrane around the bacteria.
“One of the things that bacteria need to stay functioning is their cell membrane,” she explains.
“If it starts to get holes in it or to leak, then the bacteria don’t function well and if it gets bad enough then they will die.
“One of the ways that these nanoparticles can attack bacteria is by disrupting that membrane so they make the bacteria leaky, and then things can pass in and out of the bacteria in a way they normally wouldn’t.”
The team has done tests where they’ve incorporated the nanoparticles as a coating on the surface of a medical implant, or as part of a tissue-engineering scaffold. The antimicrobial components are then gradually released into their surrounding environment, and prevent infections forming.
Presently, silver nanoparticles are often used to prevent infections, via silver-impregnated wound dressings and products. But a disadvantage of silver is its toxicity, which means its use needs to be limited. In contrast, selenium is far safer for the human body.
Associate Professor O’Connor says the selenium nanoparticles have potential for a variety of medical uses, and could help fill the gap as the efficacy of antibiotics declines.
“One of the major areas that we’re interested in is that of chronic wounds. That’s a particular concern for patients with diabetes, and increases also with the prevalence of drug-resistant bacteria,” she says.
“It has potential for other applications as well. We’re interested in potential applications in the food industry, so we’re also developing materials to help limit food spoilage and the risk of food-borne infections.”
So far, the selenium-based nanoparticles have been tested against nine different types of bacteria, and have been effective against all of them.
The team plans to file a patent within the next few months, but right now they’re wrapping up some final experiments to further understand how the nanoparticles operate.
Overall, the future looks bright for the role their discovery could play in the fight against superbugs; in fact, it might end up correcting the course that’s been unfolding since Fleming first found a way to fight bacteria.
“The benefit of the nanoparticles that we’ve developed is they attack bacteria in multiple ways,” says Associate Professor O’Connor. “It’s going to be difficult for the bacteria to develop resistance to all those different forms of attack.”
Banner image: Superbug E. coli bacteria NIAID/Flickr




